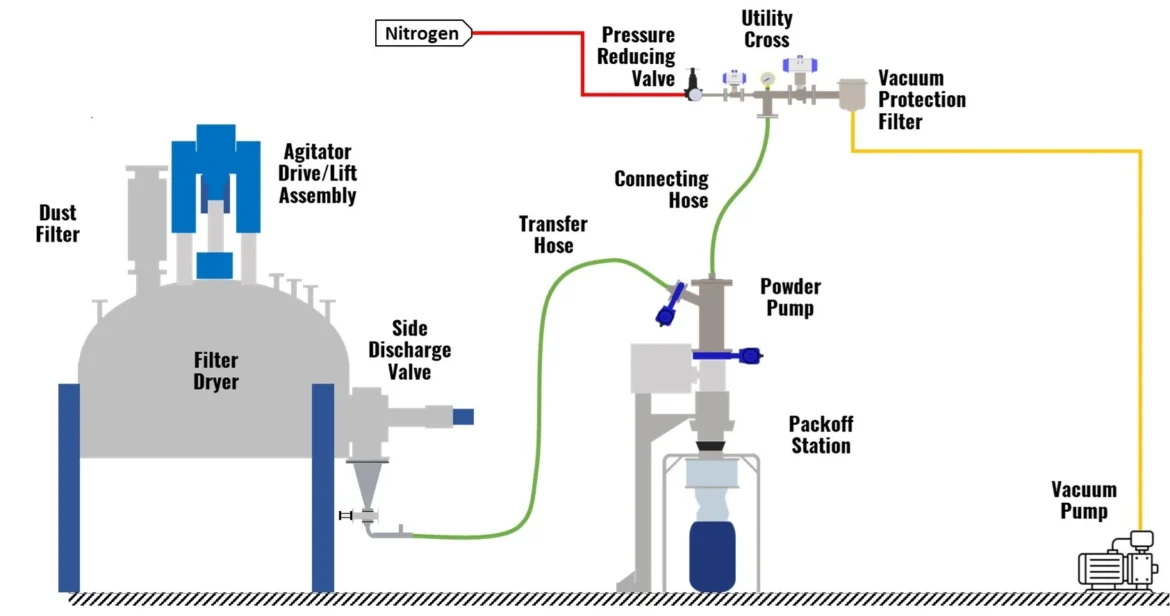
Agitated Nutsche Filter Dryers (ANFDs) are widely used in the chemical, pharmaceutical, and fine chemical industries to perform filtration, washing, and drying in a single, enclosed vessel. The performance, reliability, and safety of these systems are heavily influenced by the quality of manufacturing. To ensure consistent performance and compliance with regulatory standards, Agitated Nutsche Filter Dryer manufacturers implement rigorous quality control practices at every stage of production. These practices encompass material selection, fabrication, assembly, testing, and documentation.
An Agitated Nutsche Filter Dryer Manufacturer is responsible for delivering robust, high-performance systems that meet the specific needs of clients while ensuring safety, efficiency, and regulatory compliance. For industrial-grade systems with proven reliability and adherence to stringent quality standards, you can explore this Agitated Nutsche Filter Dryer Manufacturer, known for its emphasis on quality and precision engineering in chemical and pharmaceutical applications.
Material Verification and Selection
Quality control begins with material verification and selection. High-quality ANFDs require materials that can withstand chemical exposure, mechanical stress, and thermal cycling. Stainless steel is the standard choice for most vessels, but certain applications require specialized alloys like Hastelloy, titanium, or duplex stainless steel.
Manufacturers must verify the chemical composition, mechanical properties, and certification of all raw materials. This ensures corrosion resistance, structural integrity, and compliance with standards such as ASME for pressure vessels. Any deviation from specified material standards can compromise performance, safety, and longevity of the ANFD.
Fabrication and Welding Standards
The fabrication process is a critical area where quality control is strictly enforced. Manufacturers adhere to precise welding standards to maintain the structural integrity of the vessel and its components. Welds are inspected for defects such as porosity, cracks, or incomplete fusion using non-destructive testing methods, including X-ray, ultrasonic, or dye penetrant testing.
Additionally, vessel interiors are polished or treated to ensure smooth surfaces, which are essential for cleanability, sanitary operation, and preventing contamination in pharmaceutical and food-grade applications. Consistency in fabrication reduces the risk of leaks, mechanical failures, and maintenance issues during operation.
Component Quality and Assembly
An Agitated Nutsche Filter Dryer includes various critical components such as filter plates, agitators, seals, gaskets, heating jackets, and vacuum systems. Quality control ensures that each component meets design specifications and tolerances.
- Filter Plates and Media: Inspected for flatness, porosity, and surface finish to guarantee efficient filtration.
- Agitators: Checked for correct alignment, blade design, and material compatibility with the processed product.
- Seals and Gaskets: Verified for chemical resistance and vacuum integrity.
- Heating and Vacuum Systems: Tested for proper operation and temperature/pressure control.
During assembly, components are carefully fitted and aligned according to design specifications. Any deviation is corrected before the vessel is sealed, ensuring consistent performance during operation.
Functional Testing
Once assembled, ANFDs undergo rigorous functional testing to verify their performance under operating conditions. This may include:
- Pressure and Vacuum Testing: The vessel is subjected to vacuum and pressure conditions to confirm structural integrity and leak-tightness.
- Agitator Operation: The agitator is tested under load conditions to ensure smooth rotation, proper mixing, and alignment.
- Heating System Evaluation: Heating jackets and plates are monitored to ensure even temperature distribution and precise control.
- Filtration Trials: Some manufacturers perform trial runs using test slurries to confirm filtration efficiency, cake formation, and washing performance.
Functional testing ensures that the ANFD will perform reliably and efficiently in real-world production scenarios.
Documentation and Traceability
A robust quality control system includes comprehensive documentation and traceability. Manufacturers maintain detailed records of material certificates, fabrication processes, inspection reports, test results, and calibration records. This documentation is essential for regulatory compliance, especially in pharmaceutical and food industries that follow cGMP, FDA, or ISO standards.
Traceability allows manufacturers and clients to track each component and process step, providing assurance that the ANFD meets quality standards and facilitating corrective actions if any issues arise.
Maintenance and After-Sales Support
Quality control extends beyond the manufacturing process. Reliable manufacturers provide guidance on maintenance schedules, cleaning procedures, and replacement of critical components. Proper maintenance ensures long-term operational reliability, reduces downtime, and maintains consistent product quality.
After-sales support, including technical assistance and spare parts availability, is an integral part of quality assurance. Manufacturers who prioritize customer service demonstrate commitment to the performance and longevity of their ANFDs.
Conclusion
Quality control practices among Agitated Nutsche Filter Dryer manufacturers are essential for delivering reliable, efficient, and safe equipment. From material selection and fabrication to component inspection, functional testing, and documentation, rigorous quality control ensures that ANFDs perform optimally under demanding industrial conditions. Comprehensive quality assurance, coupled with maintenance guidance and after-sales support, not only safeguards operational efficiency but also ensures compliance with industry regulations. By choosing a manufacturer with strong quality control practices, companies can secure high-performance ANFDs that enhance productivity, product quality, and long-term reliability in chemical, pharmaceutical, and fine chemical processes.